From B. D. Walsh 25 March 1868
The Practical Entomologist, | Editor’s Office, | Rock Island, Ill.,
March 25 1868
Chas— Darwin Esq.
Dear Sir,
I should have answered your letters of Feb. 14 & 17 sooner,1 but for bad health & being busy seeing my “Report” through the Press. I expect my copies of it tomorrow or next day, when I shall do myself the pleasure of sending you one.2
After rummaging through my Journals & my entire Collection of Insects, I find but very little indeed that will answer your purpose. Such as it is, however, I enclose it. I always distrust my memory in such matters.
I do not believe that with Insects there is anything deserving the name of “attachment” or “love”. The ♂ seeks the ♀ for copulation, & so soon as that process is accomplished, the partnership is dissolved. Even with some of the social Hymenoptera, (the Honey-bees) the ♂♂ are killed off as soon as their services can be dispensed with; & with the rest of them they die off. The only exception that I have heard of—& I must say that I am rather skeptical about it—is in the genus Tomicus (Scolytidæ in Coleoptera), where European authors say that the sexes unite in boring galleries under the bark, which are afterwards stocked with eggs by the females.3 I do not doubt the fact of the males boring these galleries, but I believe that they do it simply for food. Our Apple-twig Borer (Bostrichus bicaudatus, Say)
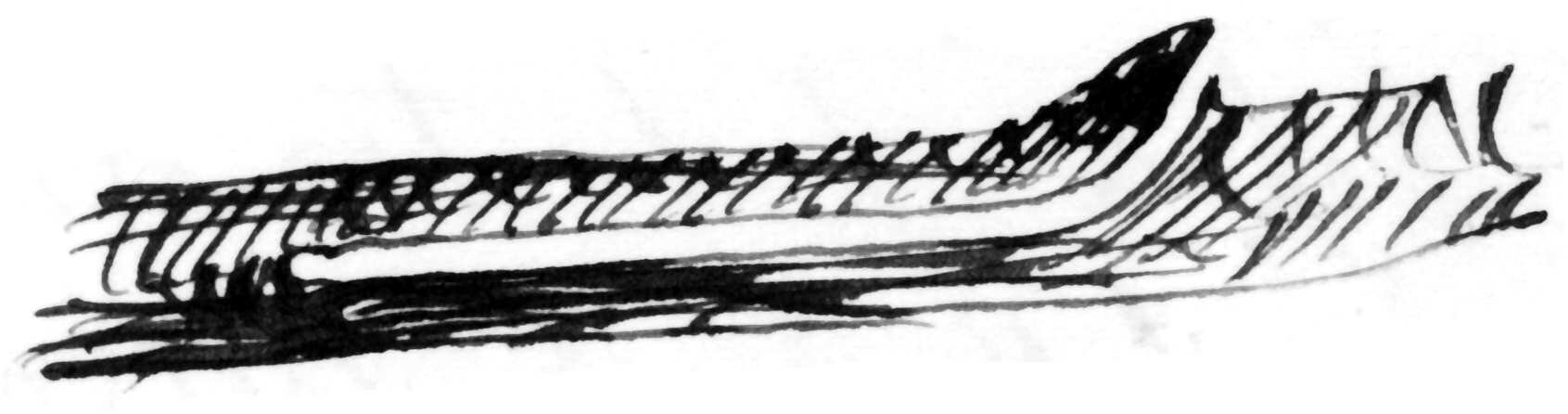
bores the pith of apple-twigs exclusively for food, no eggs being laid therein by ♀; & ♂♀ occur promiscuously, a single individual in a single boring, which is always commenced just above a bud.4
I doubt if there be any use for “the strange horns in ♂ Lamellicorns”, any more than for the tuft on the breast of ♂ Turkey, which you have referred to.5 In the Æschna family, there are earlets (“oreillettes”) one on each side of the basal abdomenal ♂ joint

(especially in the genus Gomphus,) which seem to be equally destitute of use.6 At least I can conceive no possible use for them. Gomphus is characterized by the eyes being almost as wide apart as in Agrion, & by the coloration being always black & yellow. G. vulgatissimus is an English species. In this group the tip of abdomen is clavate. The sexual distinctions in this genus are by no means conspicuous.7 I have enumerated such as occur in the species which have been described by me. The ♂♂ are by no means more brilliantly colored than ♀ ♀.
Westwood (Introd. II, p. 206) notices the fact of the male Ammophila (Sphegidæ, Hymenoptera) “being exceedingly ardent during their amours, seizing their partners round the neck with their sickle-shaped jaws”. I have noticed the same thing myself a hundred times. He also notices (p. 205) that the jaws ♀ are used in burrowing.8 This is a case of the jaws being used for two distinct purposes; & in this genus jaws ♂♀ are alike or very nearly so.
I must confess I was surprised, on looking through my collection, to find so few conspicuous colorational distinctions in the sexes. Of course there are plenty of such distinctions which are of an inconspicuous character; but these you did not want.
On the whole, I doubt whether you can make out much in support of “Sexual Selection” from what we see with our eyes of Insects. I have little doubt that the principle is found or rather exists there; but it will perhaps always be hidden from us by the wide difference in our organs of perception. We may reason by analogy from the eye of a bird or even of a fish to that of a man; but we can scarcely do so from what is conventionally called the eye of an Insect to our own eyes.9
Yours ever very truly, | Benj. D. Walsh
P.S. The questions about “Expression” are altogether out of my line. We have no Red Indians here.10
When not otherwise specified in the enclosed paper, the sexes are equal in numbers, so far as I know.
[Enclosure]
Order Neuroptera—Agrionidæ.11
1. Calopteryx maculata has black wings ♂ ♀, ♂ deep opaque black, ♀ semitransparent smoky black. The ♀ (but not ♂) has a very conspicuous white “pterostigma” (as it is called) at the tip of each wing12

2. In the very extensive N.A. genus Hetærina, ♂ (not ♀) has a beautiful carmine-red (or in some species brown) spot at the base of the wing. I enclose a ♂ specimen of H. tricolor Burm. (=rupamnensis Walsh), that you may see it for yourself. The males much the more numerous.13
Neuroptera—Æschnidæ 14
1. In Æschna clepsydra Say & A. constricta Say (two very closely allied species) the ground-color of ♂ abdomen is sky-blue, that of ♀ abdomen is pale grass-green.15 The difference is obvious, when they are on the wing.
2. In Anax junius 16 ♂ the ground color of the basal parts of the abdomen is a most vivid ultramarine blue, in ♀ grass-green; in ♂ ♀ towards the tip of abdomen the ground-color becomes a dull pale lilac or purple. When on the wing, the coloring ♂ is very conspicuous.
Neuroptera—Libellulidæ 17
1. In Plathemis 3-maculata Degeer18 the dark spots on the wings ♂ ♀ (both front & hind wings) differ, as per margin, the rest of wing being hyaline. There is never any the least hyaline spot at the tip of ♀ wing.
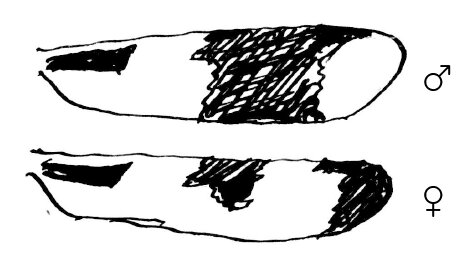
2. Both in the above species, in Libellula luctuosa Burm., in Mesothemis simplicicollis Say, & in Mes. longipennis Burm. the body of the mature ♂ becomes overspread with an external secretion of a milky-blue color on different parts of it, so as to be very conspicuous, especially in Pl. 3-maculata. Technically, this is known as “pruinescence!” & occurs also in some Agrion ♂ when mature.19 The immature ♂ differs in coloration in no respect from ♀ in all these insects.
Neuroptera—Psocidæ.20
About the enormous preponderance of ♀ ♀ in certain European Psocas, see Hagen in Proc. Ent. Soc. Phil., Oct. 1863, p. 168. (My Paper.)21
Neuroptera—Sialidæ 22
In reference to the prehensile jaws of the Lucanidæ, which by the way I do not doubt may be used for pugnacious as well as amatory purposes, I have the following remarks in No. 10 Vol. II of the Practical Entomologist, p. 10723
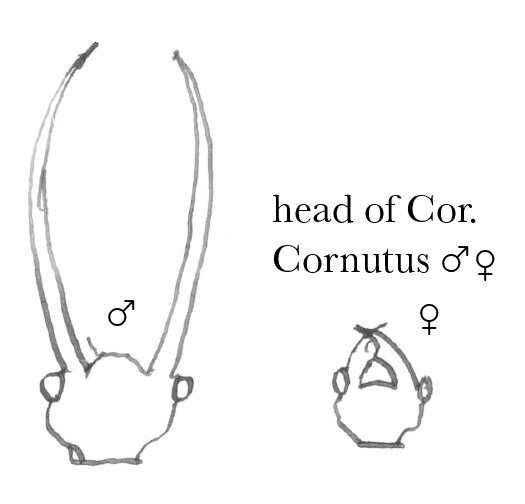
“We find another such case in a gigantic Fly with 4 gray wings, common near large rivers, (Corydalis cornutus, Linn.),24 where the jaws of the male are lengthened into the shape of the finger of a grain-cradle, evidently to enable him to embrace the soft body of the female; for, as jaws to bite or gnaw with, they are absolutely useless. In this instance, although the jaws, both of the perfect female fly & of the larva are armed with strong horny teeth, yet the jaws of the perfect male fly are quite smooth. The reason is evident. If they were armed with sharp prongs & teeth, they would penetrate the soft body of the female fly, & thus defeat the purpose for which nature constructed them. The Horn-bugs, on the contrary, are, as is well known, enveloped in a strong coat of mail; & here we find the prehensile jaws of the male armed with sharp prongs & teeth, & curved in such a manner as to give the best possible grip on the slippery shelly body of the female”.
Orthoptera—Phasmidæ.25
The only conspicuous colorational distinction that I notice in this Order is Spectrum (diapheromera) femoratum Say;26 where ♂ is of a polished, shining brownish-yellow color, & ♀ of a dull, opaque, cinerous-brown color, when mature, but when immature ♂ ♀ are green.
Coleoptera—Crioceridæ 27
Orsodacna atra Ahrens is black, legs & all; O. ruficollis Newman differs in having a rufous thorax. The former is the ♂, the latter the ♀, of one & the same species, & I have found them repeatedly in coitu.28
Coleoptera—Cryptocephalidæ 29
In Anomoia laticlavis Forster, the ♂ has the anterior legs greatly elongated, & the tips of the front tibiæ crooked inwards, doubtless for sexual purposes.30
Hymenoptera—Cynipidæ.31
Almost Universally, so far as I know, in the gall-making, species of Cynips the ♀ ♀ are at least 4 or 5 times as numerous as ♂ ♂; & in certain species there appear to be no ♂ ♂ at all, at least in ordinary seasons. So in the allied genus Rhodites, making galls on the Rose.32 In certain species the ♂ ♂ are very rare indeed. On the other hand, in the inquilinous Gall-flies, the sexes are about equal.
Hymenoptera—Tenthredinidæ 33
Curtis says “It generally happens that the female saw-flies are much less abundant than the other sex, & this is believed to be the case with the Turnip-sawfly (Athalia spinarum), the males being as 6 to 1 when they have been bred; but it has been exactly the reverse in those I have caught in the fields, for out of 15 specimens there were only 3 males”. (Farm Insects, pp. 45–6)34 Probably it was a little late in the season; for it is a very general rule with Insects that ♂ comes out 2 or 3 days, or even 2 or 3 weeks, before ♀. Edwards35 finds this to be so with almost all Butterflies; & I have noticed it in many of the orders. For example, June 7 & 8 I bred 86 ♂ of a gall-making Gallfly, Cynips q. operator O.S.,36 without a single ♀ among them; June 9 85 ♂, & only 4 ♀; June 10, out of 54 counted promiscuously, 52 were ♀. Sometimes (& I think generally with us) in the Sawflies the ♀ ♀ are the most numerous. Of Pristiphora grossulariæ Walsh (the N.A. gooseberry sawfly) I bred 4 ♂, 49 ♀; & of Cræsus latitarsus Norton I bred about 4 times as many ♀ as ♂37
Hymenoptera—Uroceridæ 38
In Tremex columba Linn. the ♀ is much brighter-colored than ♂, having abdomen very variably banded with yellow, while ♂ has brown or piceous abdomen.—39
Diptera—Cecidomyidæ. 40
In the gall-making Cecidomyia the ♀ ♀ are about 4 times as numerous as ♂ ♂. The discrepancy is especially remarkable in my Cec. s. batatas,41 of which I bred hundreds of ♀ to 1 ♂. In the inquilinous Cecidomyia, there seems to be little difference in numbers.
Lepidoptera—the Butterflies.
Throughout Papilio, the ♂ ♂ are 4 times (about) as numerous as ♀ ♀. In many species the ♀ is hard to get. This is what Edwards says; & he has large experience, receiving many collections from all quarters, & collecting himself extensively. In Papilio Turnus the ♂ ♂ are certainly at least 4 times as numerous as ♀ ♀; we have only 4 species of the genus here.42
In Pieris protodice the ♂ ♂ are remarkably less marked with black than ♀ ♀; so that, when I began collecting, I took them for distinct species.43 So far as I recollect, the same law prevails with your English species. In Parnassius smintheus (the only species of the genus I have) the same thing holds good.44 Almost any one at first sight would take the ♀ for a different species.— In Hipparchia nephele (color brown) the ♂ is always several shades darker than ♀, so that I at first thought them distinct species.—45 In Nathalis iole, ♀ has a good deal more black than ♂ on upper face of hind wing, & in ♂, there is a singular oval white spot (B) enclosed in the black band on the upper face of the hind wing, so as to be generally concealed by the overlap of the front wing, which is not found in ♀.46 On the other hand, in Colias philodice, C. eurytheme, & C. cæsonia, ♂ has more black in both wings above than ♀; & I believe it is the same in your European species, Edusa & hyale.—47 In Danais archippus ♂ has a singular black spot (A) by the side of one of the veins of the hind wing, which is not found in ♀.48
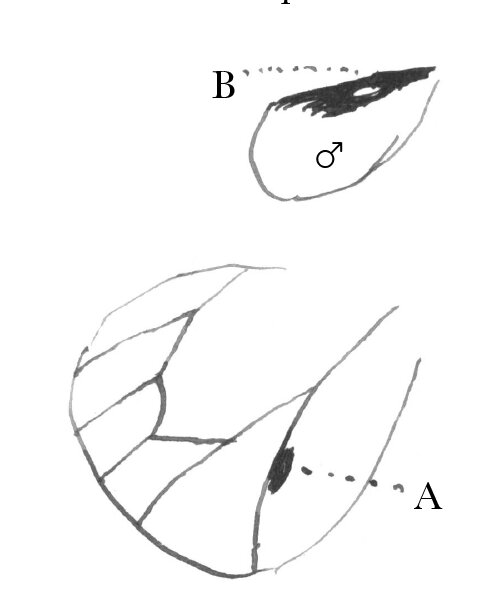
In Thecla falacer? Bdv. & Lec. the ♂ has an oval spot of a paler brown color & shining, on the middle of the subcosta above, which is absent in ♀.— In Thecla poeas? Hübn., one sex (I think ♀) is mostly bright blue above, the other brown.—49 In Polyommatus neglectus & P. pseudargiolus, ♂ wings are blue immaculate above, ♀ broadly edged with black except behind.50 In P. comyntas Godt., P. lygdamas Doubl., P. heteronea Bdv., & P. acmon Doubl., ♂ wings are blue above, ♀ brown (more or less tinged with blue) or reddish brown.—51 Argynnis Diana, you know all about the coloration; as to the numbers of the sexes, I think that Edwards found them about equal.52 At all events, he told me nothing to the contrary.
Lepidoptera–the Moths.
In Cossus robiniæ 53 the top half of the hind wing is orange not uniform dull gray as in ♀.— In Ægeria exitiosa 54 Say. ♀ there is a broad orange band on middle of the steel-blue abdomen not found in ♂; but ♂ front wing is mostly hyaline & that of ♀ steel-blue opaque.— In Saturnia io ground-color of ♂ wings is bright ochre-yellow, instead of reddish brown as in ♀.—55 In Acontia (2 species) a genus of Tineidæ,56 ♀ has a good deal more black than ♂.
CD annotations
Footnotes
Bibliography
Correspondence: The correspondence of Charles Darwin. Edited by Frederick Burkhardt et al. 29 vols to date. Cambridge: Cambridge University Press. 1985–.
Curtis, John. 1860. Farm insects: being the natural history and economy of the insects injurious to the field crops of Great Britain and Ireland, and also those which infest barns and granaries, with suggestions for their destruction. Glasgow: Blackie and Son.
Descent: The descent of man, and selection in relation to sex. By Charles Darwin. 2 vols. London: John Murray. 1871.
Edwards, William Henry. 1864. Description of the female of Argynnis Diana. Proceedings of the Entomological Society of Philadelphia 3: 431–4.
Emmet, A. Maitland. 1991. The scientific names of the British lepidoptera: their history and meaning. Colchester: Harley Books.
Garrison, Rosser W. 1990. A synopsis of the genus Hetaerina with descriptions of four new species (Odonata: Calopterygidae). Transactions of the American Entomological Society 116: 175–259.
Kirkendall, Lawrence R. 1983. The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zoological Journal of the Linnean Society 77: 293-352.
Montgomery, B. Elwood. 1954. Nomenclatural confusion in the Odonata: the Agrion–Calopteryx problems. Annals of the Entomological Society of America 47: 471–83.
Morris, John Gottlieb. 1862. Synopsis of the described Lepidoptera of North America. Part 1: diurnal and crepuscular Lepidoptera. Smithsonian Miscellaneous Collections, vol. 4. Washington, D.C.: Smithsonian Institution.
OED: The Oxford English dictionary. Being a corrected re-issue with an introduction, supplement and bibliography of a new English dictionary. Edited by James A. H. Murray, et al. 12 vols. and supplement. Oxford: Clarendon Press. 1970. A supplement to the Oxford English dictionary. 4 vols. Edited by R. W. Burchfield. Oxford: Clarendon Press. 1972–86. The Oxford English dictionary. 2d edition. 20 vols. Prepared by J. A. Simpson and E. S. C. Weiner. Oxford: Clarendon Press. 1989. Oxford English dictionary additional series. 3 vols. Edited by John Simpson et al. Oxford: Clarendon Press. 1993–7.
Silsby, Jill. 2001. Dragonflies of the world. London: Natural History Museum in association with CSIRO Publishing.
Stainton, Henry Tibbatts. 1856–9. A manual of British butterflies and moths. 2 vols. London: J. van Voorst.
Variation: The variation of animals and plants under domestication. By Charles Darwin. 2 vols. London: John Murray. 1868.
Walsh, Benjamin Dann. 1868. First annual report on the noxious insects of Illinois. Chicago: Prairie Farmer Steam Print.
Westwood, John Obadiah. 1839–40. An introduction to the modern classification of insects; founded on the natural habits and corresponding organisation of the different families. 2 vols. London: Longman, Orme, Brown, Green, and Longman.
Summary
Sexual preference in insects;
structures for seizing females;
coloration.
Doubts whether CD can make much of a case from insects in support of sexual selection.
Letter details
- Letter no.
- DCP-LETT-6051
- From
- Benjamin Dann Walsh
- To
- Charles Robert Darwin
- Sent from
- Rock Island, Ill.
- Source of text
- DAR 82: A90–1; A117–18, DAR 85: B65
- Physical description
- ALS 4pp † encl 4pp
Please cite as
Darwin Correspondence Project, “Letter no. 6051,” accessed on
Also published in The Correspondence of Charles Darwin, vol. 16


